|
Probe in plastic storage case |
FID Head |
|
|
Module |
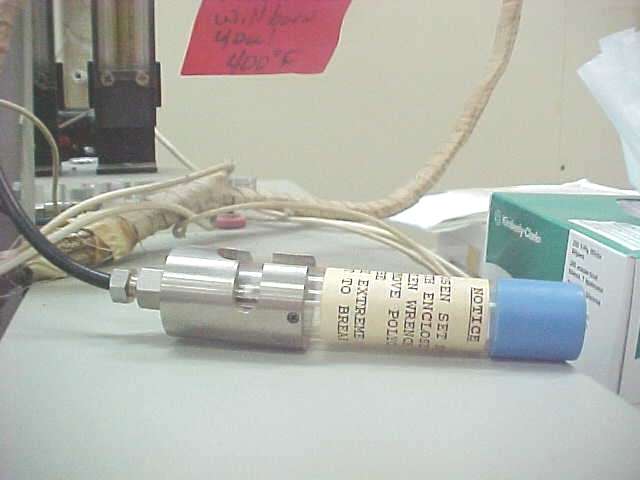
Note the basic violation of lab policy. Sample, even though in a sealed vial, setting on top of an instrument. If spilled this will destroy paint and attack the electronics. |
The probe fits loosely over the top of the FID with a cutout for the signal cable. The Allen wrench has been left in place in this picture to remind that the Allen set screw must be snug to prevent vibrations and thereby reduce signal noise. |
|
Initial Value |
100° C |
Initial Time Minutes |
2.00 |
|
Rate |
5.0 Deg./Min |
Final Value |
270°C |
|
Final Time Minutes |
5.00 |
Injector B |
250°C |
|
Detector B |
250°C |
Equilb Time Minutes |
1.00 |
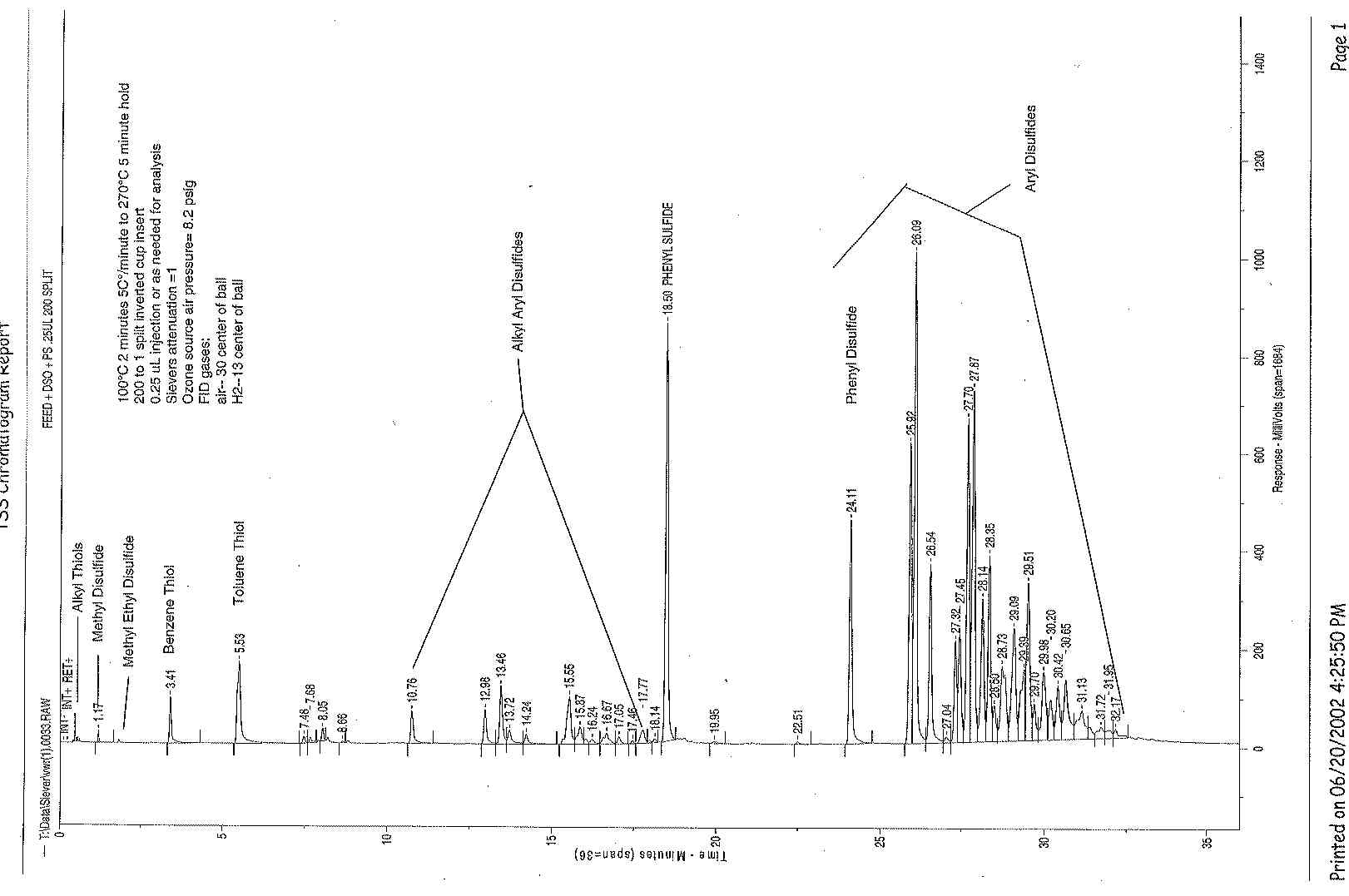
Supply Gas Settings are:
|
Head Pressure |
15 psi |
|
H2 Top of Ball |
15 |
|
Air Top of Ball |
34 |
|
Mercaptan Sulfur by titration Wt% |
Grams Phenyl Sulfide to add to 10 grams Crude Acid |
Grams Phenyl Sulfide to add to 50 grams Crude Acid |
|
0.02 |
0.012 |
0.060 |
|
0.05 |
0.029 |
0.145 |
|
0.10 |
0.058 |
0.290 |
|
0.15 |
0.087 |
0.435 |
|
0.20 |
0.120 |
0.600 |
|
0.25 |
0.145 |
0.725 |
|
0.30 |
0.174 |
0.870 |
|
0.35 |
0.203 |
1.015 |
| . | Inland 19 | Invoil 20 | Inland 45 | Inland TW | Fomplin Y06/6 |
| Vapor Pressure | 3 x 10-5 torr | 3 x 10-6 | 1 x 10-7 | 1 x 10-6 | 3 x 10-6 |